ELMED keeps all the spare parts required for the medical devices it manufactures ready in its stock and, if needed, quickly intervenes in the defective device and fixes the malfunction.
ELMED manufactures consumables for the medical devices it produces. Compliant with medical device legislation, biocompatible, high quality and economical. Always use original spare parts and consumables in your device to get high performance like the first day.
Product Security Statement
This paper summarizes the ELMED Medical Systems position on securing our products, services, applications, and systems and describes our processes for providing products with Security Designed In.
Background
We at ELMED Medical Systems recognize that the security of ELMED Medical Systems NICU products and services are an important part of our security planning. We are dedicated to helping you maintain the confidentiality, integrity, and availability of personal data, business data, and the ELMED Medical Systems hardware and software products that create and manage this data.
Threats to the security of devices and personal and healthcare information continue to increase. These threats include malicious security attacks via viruses, worms, and hacker intrusions.
To fulfill our commitment to security, we at ELMED Medical Systems maintain a cyber security program to: develop, deploy, and support advanced security features for our products and services.
Manage security events in the field. ELMED Medical Systems participates in industry and government collaborations to help ensure product innovations and clinical information is produced and available at the highest level of quality, availability, and confidentiality.
We implement security within a heavily regulated medical device industry. Government regulations (e.g., those of the US Food and Drug Administration (FDA), European Commission) require that hardware and software changes be subjected to rigorous verification and validation to ensure high safety and performance standards are met in all ELMED Medical Systems medical devices.
Likewise, ELMED Medical Systems strives to ensure that same high standard for NICU products and services. Organization ELMED Medical Systems operates under a Product Security Policy governing Security Designed In creation of products and services.
The policy also governs risk assessment, identification of vulnerabilities in existing products, and incident response activities.
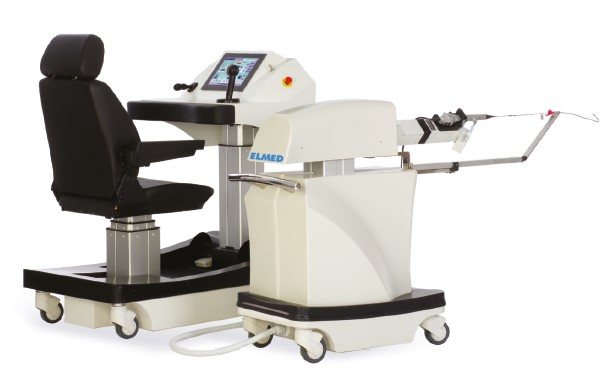
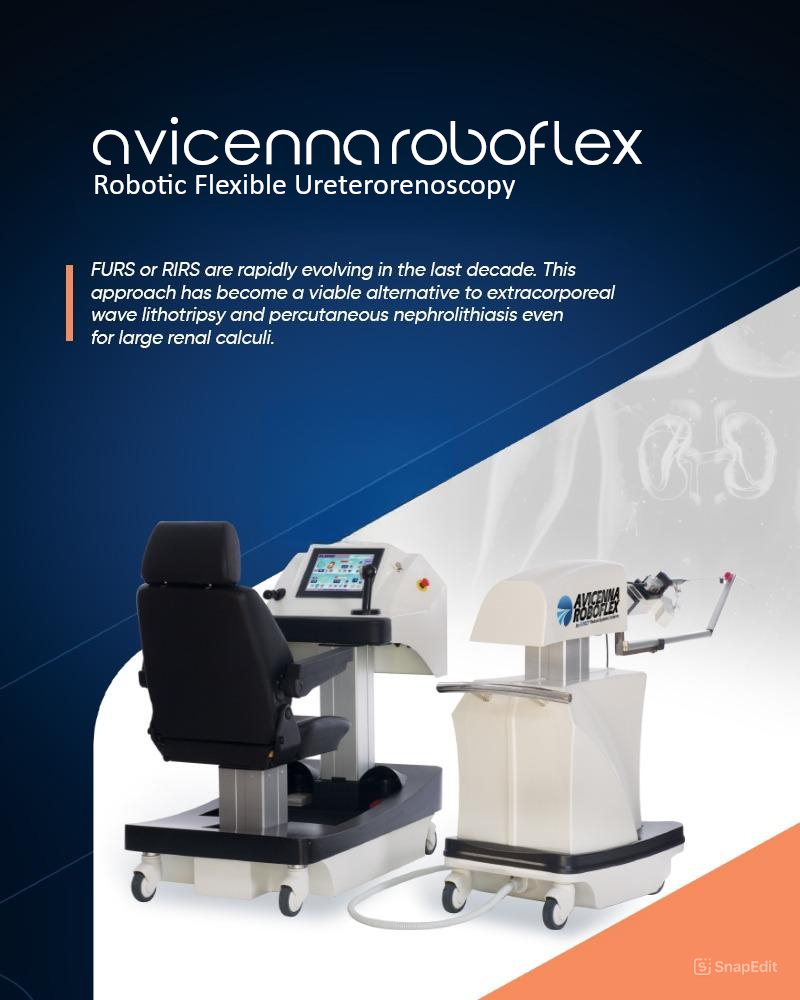
Avicenna Roboflex provides protection for endoscope, precision for the stone treatment, ergonomics for the surgeon. Innovative robotic solution for flexible ureteroscopy. The Avice...
Product Details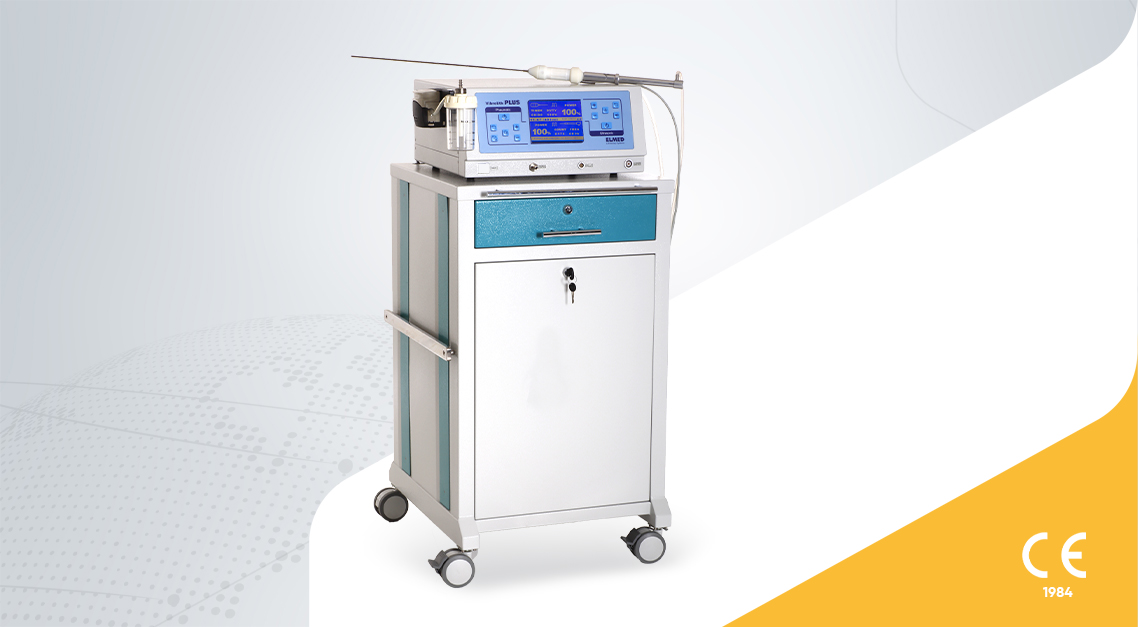
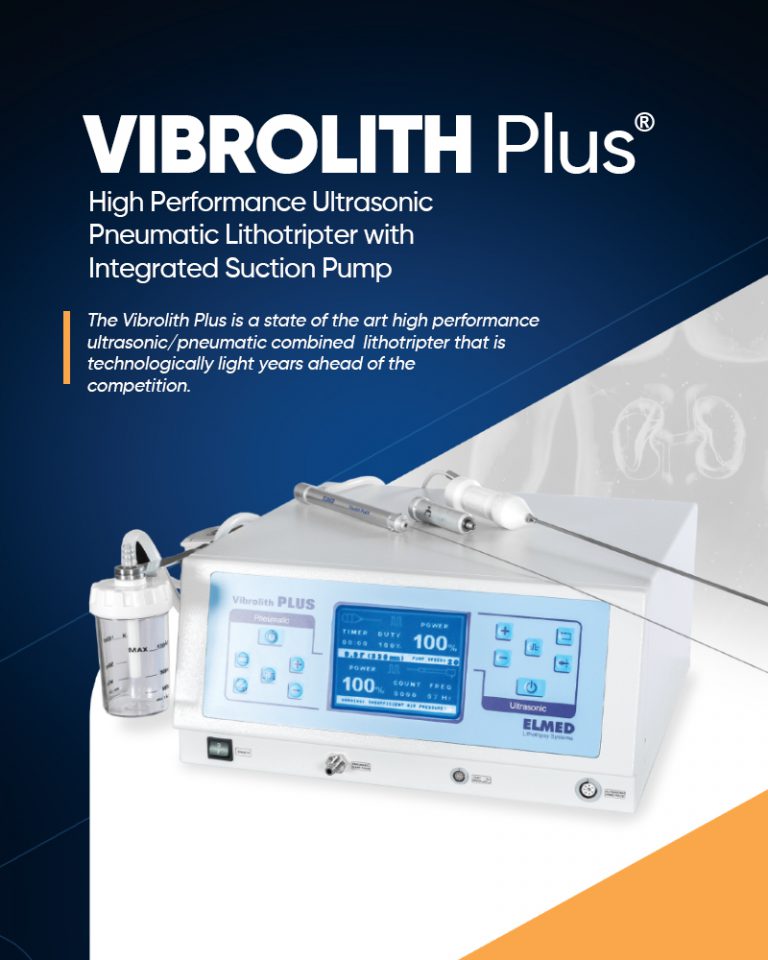
VIBROLITH Plus is an intracorporeal pneumatic ultrasonic combined lithotripter for percutaneous lithotripsy that combines the high efficiency of the electro-pneumatic lithotripter ...
Product Details
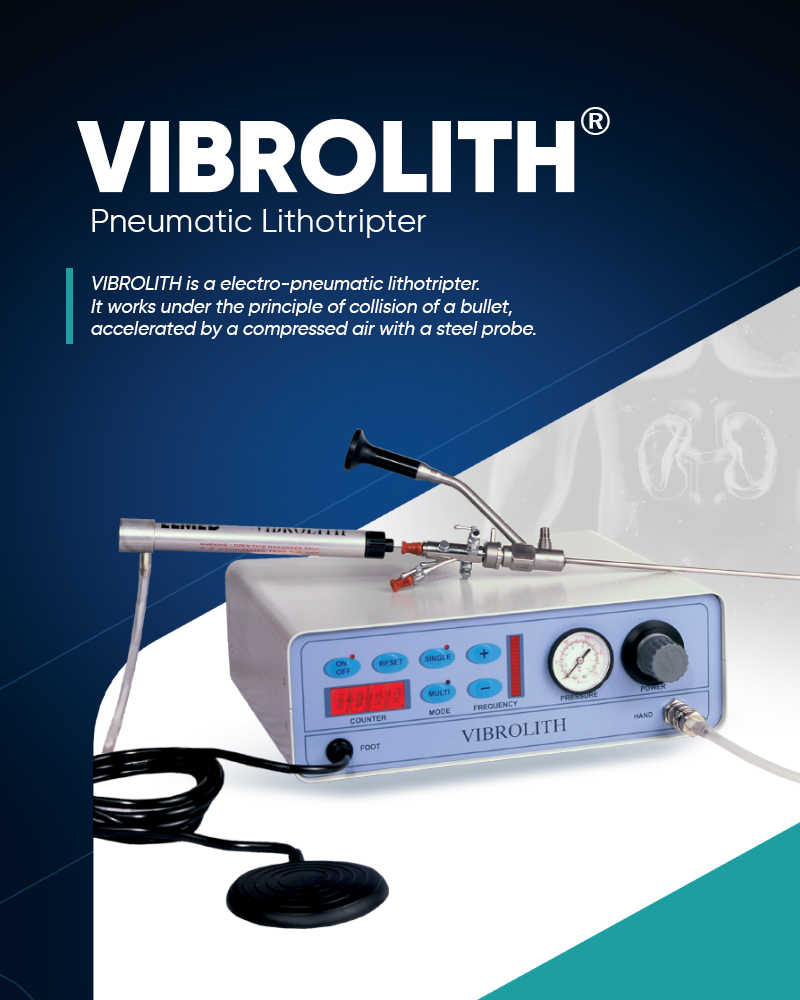
VIBROLITH is an effective intracorporeal pneumatic lithotripter designed for powerful and rapid treatment of urinary tract stones in kidney, ureter and bladder using ballistic shoc...
Product Details
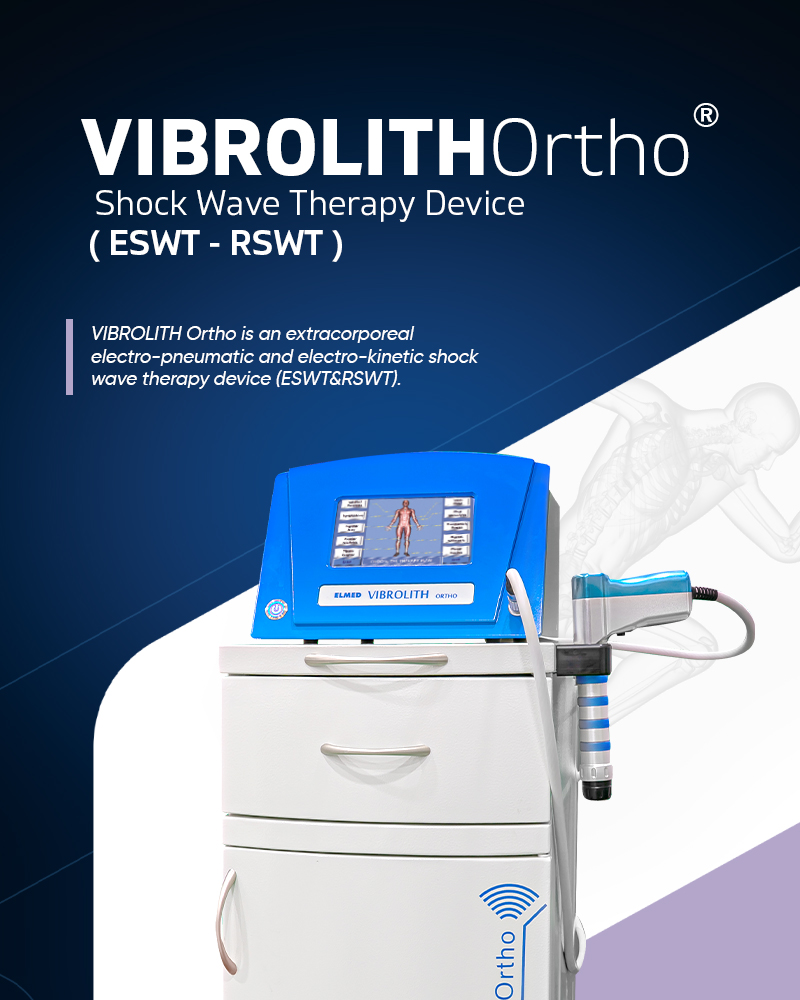
VIBROLITH Ortho is a therapy device that provides healing in soft tissues by transmitting pneumatically sourced ultrasonic waves radially to the application area with the applicato...
Product Details